School of architecture, building and design bachelor of quantity surveying (honours) site surveying qsb 60103 field work 1 report levelling darren tan quan wen 0322662 yeap phay shian 0322243 lee xin ying 0322432 michelle tung man kaye 0324175 loh mun tong 0323680 lecturer: mr. Students in Surveying and Mapping Science at East Tennessee State University pursue coursework leading to professional land surveying practice and successful completion of the NCEES Fundamentals of Surveying exam. Graduates from this program are well prepared for future licensing in any state and for occupying professional level positions with. Program Details. The land surveying & mapping technology program prepares students to enter a high-tech profession that uses state-of-the-art equipment to determine the location and measurement of improvements and other physical features above or below the earth s surface. Surveying is an integral component for land development by civil engineers, municipal planners, and the construction industry.
Land Surveying Certificate Program Highlights
UW’s 100 percent online land surveying certificate program makes it possible for you to earn the credential that will help you improve your skills, while also offering the education you need to become a licensed land surveyor in states that don’t require ABET certification.
Here are some additional reasons to choose UW:

Distance learning delivery. Maintain your current work schedule. UW’s distance learning format consists of downloadable, pre-recorded lectures as well as weekly teleconferences with instructors. Exams are typically take-home with the exception of a proctored final exam.
Scholarships. Take advantage of scholarship opportunities for land surveying students. Also, check with your employer to see whether your company will fund all or part of your land surveying certificate.
Faculty. Learn from faculty who have professional experience as land surveyors and can help you apply new concepts immediately, if you are currently working in the field.
Great job prospects. Enjoy a bright future. UW land surveying students are often offered jobs before they graduate
- Docket Number:
- FDA-2014-D-0435
- Issued by:
DRAFT GUIDANCE
This guidance document is being distributed for comment purposes only.
Document issued on: May 5, 2014
You should submit comments and suggestions regarding this draft document within 90 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit written comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. Submit electronic comments to http://www.regulations.gov. Identify all comments with the docket number listed in the notice of availability that publishes in the Federal Register.
For questions regarding this document contact Robert J. Doyle at 301-796-5863 or via e-mail at robert.doyle@fda.hhs.gov.
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Devices and Radiological Health
Office of In Vitro Diagnostics and Radiological Health
Division of Radiological Health
Magnetic Resonance and Electronic Products Branch
Preface
Additional Copies
Additional copies are available from the Internet. You may also send an e-mail request to CDRH-Guidance@fda.hhs.gov to receive a copy of the guidance. Please use the document number 1764 to identify the guidance you are requesting.
Surveying, Leveling, or Alignment Laser Products - Draft Guidance for Industry and Food and Drug Administration Staff

This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.
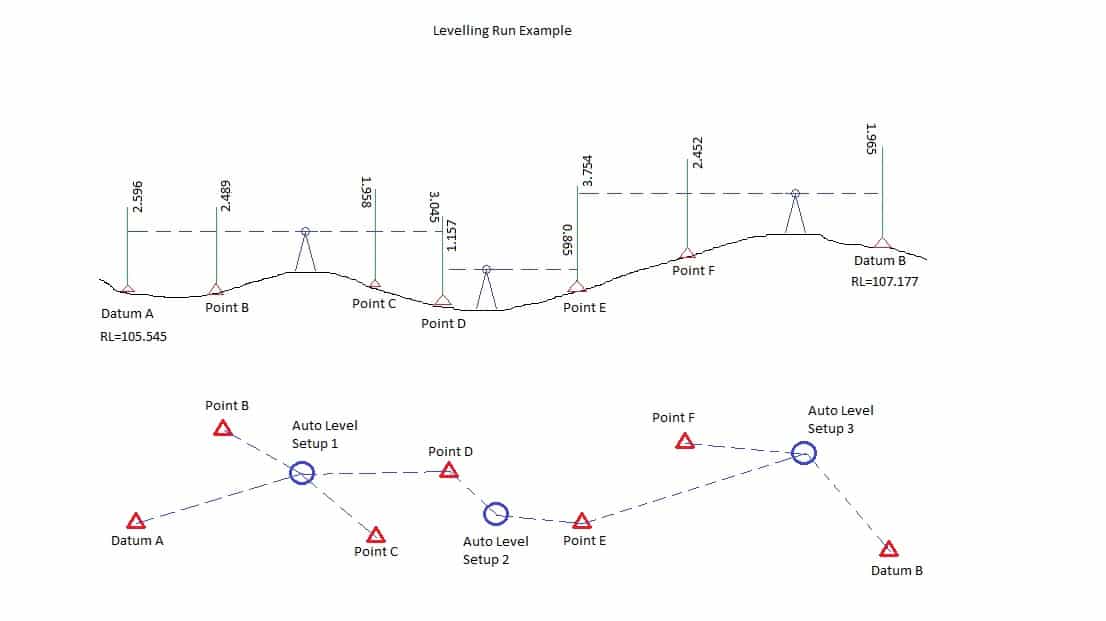
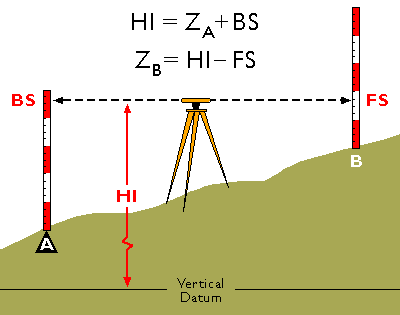
Leveling Program Surveying Training
Introduction
This draft guidance is intended for manufacturers of laser products and outlines the Food and Drug Administration’s (FDA’s or the Agency’s) proposed approach regarding the applicability of FDA’s performance standard regulations to surveying, leveling, or alignment (SLA) laser products.
The topics that are addressed include:
- The definition of an SLA laser product
- Examples of SLA laser products
- Design features of SLA laser products
- Applicability of class limits to SLA laser products
- Questions and answers relating to the application of FDA’s performance standard regulations to SLA laser products
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
Surveying, Leveling, or Alignment (SLA) Laser Products
FDA regulates radiation-emitting electronic products, including all types of lasers. The Agency sets radiation safety product performance standards that must be met by manufacturers in order for laser products to be legally sold in the U.S. market. This draft guidance is intended to provide a brief summary of the FDA’s proposed approach on the applicability of FDA’s performance standards for laser products to specific purpose SLA laser products and is not a substitute for the performance standards themselves.
1. Question: What is an SLA laser?
Answer: SLA lasers are a subcategory of specific purpose laser products that transmit laser radiation through open space for surveying, alignment, or leveling purposes. An SLA laser is defined in 21 CFR 1040.10(b)(39) as 'a laser product manufactured, designed, intended or promoted for one or more of the following uses:

- (Determining and delineating the form, extent, or position of a point, body, or area by taking angular measurement.
- Positioning or adjusting parts in proper relation to one another.
- Defining a plane, level, elevation, or straight line.'
It is important to note that a laser product can fall under the SLA laser definition even if it is not promoted for one of the three uses listed in the SLA laser definition or is explicitly promoted for a different use as long as the laser product is manufactured, designed, or intended for one or more of those SLA laser uses.
2. Question: What are some examples of SLA laser products?
Answer: Examples of products that FDA is aware of that are designed and manufactured for, if not also intended, or promoted for, one or more of the uses listed in 21 CFR 1040.10(b)(39), include but are not limited to:
- Laser pointers1
- Levels
- Tools incorporating laser guides
- Gun sights
- Target designators
- Night vision illuminators
- Visual disruptors
Leveling Program Surveying Guide
3. Question: What design features does CDRH consider specific to SLA lasers?
Answer: Certain design features allow SLA lasers to be used in open spaces or in unrestricted environments to determine and delineate the form, extent, or position of a point, body, or area by taking angular measurement, position or adjust parts in proper relation to one another, or define a plane, level, elevation, or straight line. These design features include:
- Compact size (i.e. small, lightweight)
- Battery power
- Ergonomic design to permit hand-held use
- An aperture in the laser product's protective housing to transmit laser emission into open space
- Portability to permit use in open spaces or in unrestricted environments
- Features that utilize the laser’s straight line emission for surveying, leveling, or alignment
Generally, FDA will consider these design features as evidence that the laser product was designed for one or more of the uses listed in the SLA laser definition at 21 CFR 1040.10(b)(39). Therefore, FDA will generally consider laser products with these design features to fall under the definition of an SLA laser product and to be subject to the requirements identified in 21 CFR 1040.11(b) regardless of whether such laser products are promoted for SLA laser uses.
Design features that are not typical of an SLA laser include:
- Predictable, stable power input and output
- High quality power supply and/or power conditioning components
- Adjustability of power and wavelength
- Design that facilitates remote actuation2
- Non-portability
- Hard wire connection to power mains
4. Question: Why is a class limit imposed on SLA lasers?
Answer: The class limit in 21 CFR 1040.11(b) is intended to impose an upper exposure limit on accessible laser emission to ensure the safety of users and others. This limit takes into account the product’s intended uses and the generally unrestricted environments in which SLA laser products are used.
Note: 21 CFR 1040.11(b) establishes an upper class limit for all SLA laser products as Class IIIa, which has an accessible emission limit of 5 milliwatts.3 FDA has issued a proposed rule4 to amend the performance standards for laser products to achieve closer harmonization between the FDA’s current standards and the IEC standards. FDA has proposed that the IEC class limits be incorporated by reference into FDA’s regulations such that FDA’s class limits would be identical to the IEC class limits. Until this rule is finalized, FDA’s Center for Devices and Radiological Health (CDRH) does not intend to object to SLA laser emissions that are within the accessible emission limits for Classes 1, 2, and 3R in the International Electrotechnical Commission (IEC) International Standard 60825-1, “Safety of laser products- Part 1: Equipment classification and requirements,” Ed. 3.0 (IEC 60825-1) at Tables 3-7, since these are very similar to the class limits for SLA lasers in FDA’s regulations and adequately assure safety. However, because IEC Classes 1M and 2M do not have comparable analogs in FDA’s classification system, manufacturers should not conform to the parameters for IEC Classes 1M or 2M unless they also comply with FDA’s performance standards for laser products.
5. Question: May laser product manufacturers avoid the specific-purpose SLA designation simply by promoting the lasers for scientific, general-purpose, or other uses?
Answer: No. As discussed above in the answer to Question 1, a laser product manufacturer may not avoid designation of its product as an SLA laser simply by promoting the laser product for non-SLA laser uses if the laser product is manufactured, designed, or intended for one or more of the uses listed in SLA laser definition. Therefore, promoting an SLA laser product for other purposes, such as science, treating pain, burning, security, or light reflection will not necessarily prevent the product from falling under the SLA laser definition. In determining whether a laser product was designed for one of the uses listed in the SLA laser definition, FDA looks at the design features identified in the answer to Question 3 above. The SLA designation imposes class limits on the accessible laser emission from laser products in order to promote their safe operation in generally unrestricted environments.
6. Question: When laser products have multiple purposes, which purpose will guide manufacturers in determining whether the laser product is an SLA laser?
Answer: When a laser product is manufactured, designed, intended or promoted for one of the uses listed in the definition of an SLA laser product at 21 CFR 1040.10(b)(39), the laser product will be subject to FDA’s performance standard applicable to SLA laser products even if the laser product also has non-SLA laser uses.
7. Question: Do other Federal agencies work with FDA to stop false or misleading promotions of regulated laser products?
Answer: Yes, FDA and the Federal Trade Commission work cooperatively to stop false or misleading promotions of FDA-regulated products.
1 Some laser pointers may be demonstration laser products, as defined in 21 CFR 1040.10(b)(13), if they are manufactured, designed, intended, or promoted for purposes of demonstration, entertainment, advertising display, or artistic composition. Laser pointers are subject to the same class limits regardless of whether they are classified as SLA laser products or demonstration laser products. See 21 CFR 1040.11(b) and (c).
2 See 38 FR 34084, 34085 (December 10, 1973).
3 This means that SLA lasers that emit invisible radiation (wavelengths up to and including 400 nanometers and wavelengths higher than 710 nanometers) may not exceed the accessible emission limits for Class I, because Classes IIa, II and IIIa do not include wavelengths outside the visible range.
4 78 FR 37723 (June 24, 2013).
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
Leveling Program Surveying Pdf
All written comments should be identified with this document's docket number: FDA-2014-D-0435.